The Biosimilars Market is experiencing a surge in growth, driven by the increasing popularity of generic biologics. These biologics, also known as biosimilars, offer cost-effective alternatives to branded biologic drugs, providing patients with access to high-quality treatments at reduced prices. The development of generic biologics involves a rigorous process to demonstrate similarity to the reference biologic, ensuring safety, efficacy, and quality. By offering comparable therapeutic options, biosimilars play a crucial role in expanding treatment access and improving healthcare affordability worldwide.
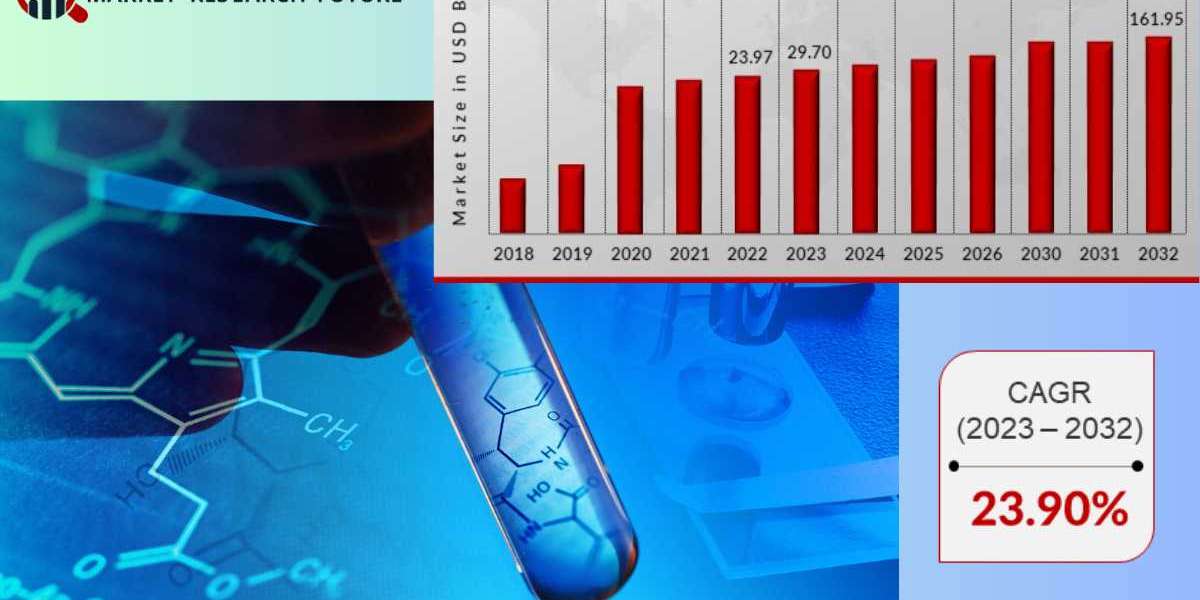
According to Market Research Future (MRFR), the biosimilars market size was valued at USD 23.97 billion in 2022 and is projected to grow from USD 29.70 Billion in 2023 to USD 161.95 billion by 2032, exhibiting a compound annual growth rate (CAGR) of 23.90% during the forecast period (2023 - 2032).
Biosimilars Market: Latest News and Developments
- The FDA has approved the first Humira biosimilar. Adalimumab-bwwd (Cyltezo), a Humira biosimilar, received FDA approval in January 2023 for the treatment of rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, juvenile idiopathic arthritis, Crohn's disease, ulcerative colitis, plaque psoriasis, and hidradenitis suppurativa.
- Rising use of biosimilars in the treatment of cancer. A growing number of biosimilars are being used to treat cancer. The FDA authorised filgrastim-sndz (Zarxio), pegfilgrastim-jmdb (Onpro), and trastuzumab-dkst (Herzuma) as three biosimilars for the treatment of cancer in 2022.
Market Segmentation:
- Product Segmentation:
- Recombinant Glycosylated Proteins
- Recombinant Peptides
- Recombinant Non-Glycosylated Proteins
- Largest segment due to soaring cases of chronic disorders
- Widely used therapeutically for their availability and cost-effectiveness
- Application Segmentation:
- Chronic Diseases
- Oncology
- Blood Disorders
- Autoimmune Diseases
- Infectious Diseases
- Growth Hormone Deficiency
- Blood disorders segment is prominent due to rising global burden and cost-effectiveness of biosimilars
- End User Segmentation:
- Hospitals and Clinics
- Research Institutes
- Hospitals and clinics are leading segments due to accessibility to treatment options and skilled professionals
Regional Status:
- Europe:
- Leading market due to large elderly population and prevalence of lifestyle-related disorders
- Expected higher market growth due to upcoming patent expiries of blockbuster biologics
- North America:
- Bright outlook with potential for lucrative growth
- Surging burden of chronic ailments and increased spending on research activities contribute to market expansion
- Favorable reimbursement landscape in the US encourages healthy competition
- Asia Pacific (APAC):
- Significant growth expected, especially in China, South Korea, and India
- Generics-driven countries with advanced manufacturing platforms leading to lower production costs
- Majority of upcoming patent expiries in biosimilars presents profitable opportunities for manufacturers
An integral component of the Biosimilars Market is the emergence of follow-on biologics. These biologics, which closely resemble existing biologic drugs, offer additional options for patients and healthcare providers. Follow-on biologics undergo rigorous testing to demonstrate similarity to the reference product, ensuring interchangeability and therapeutic equivalence. With their introduction, follow-on biologics stimulate competition in the biopharmaceutical industry, driving down prices and promoting innovation. As the demand for cost-effective biologic therapies continues to grow, the Biosimilars Market stands to benefit from the availability and acceptance of follow-on biologics, expanding treatment options and improving patient outcomes.
Eminent Vendors
Top global biosimilars Companies include Stada Arzneimittel AG (Germany), Teva Pharmaceuticals (Israel), Biocon (India), Pfizer (US), Sandoz International (Germany), Eli Lily Company (US), Actavis, Inc. (US), Dr. Reddy’s Laboratories (India), Cipla Ltd (India), Amgen, Inc. (US), Samsung Biologics (South Korea), Hospira Inc.(US), Mylan, Inc.(US), Celltrion (South Korea), to mention a few.
For more information visit at MarketResearchFuture
Other Trending Reports