Key Market Insights
- Presently, over 120 AI software and service providers claim to offer AI products / technologies for clinical trials to multiple end-users; over 90% of the players are headquartered in developed geographies.
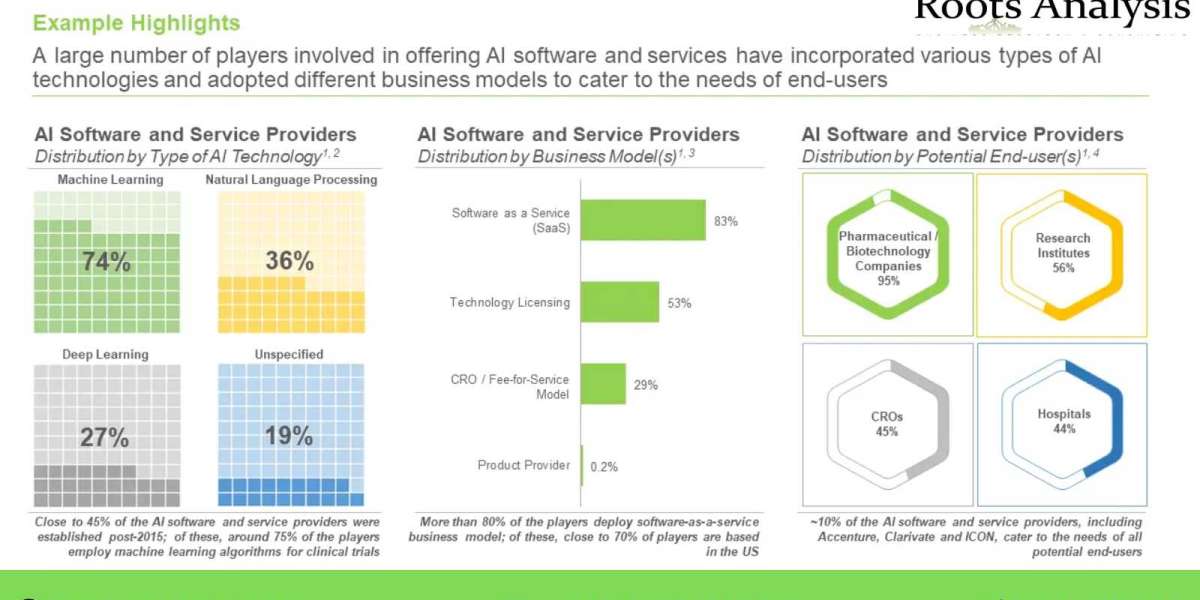
- A large number of players involved in offering AI software and services have incorporated various types of AI technologies and adopted different business models to cater to the needs of end-users.
- The annual number of clinical studies, based on AI, has steadily evolved; this indicates the growing adoption of AI solutions by pharmaceutical and biotechnology companies, hospitals, research institutes and CROs.
- The rising interest is also evident from the partnership activity; more than 55% of the deals have been focused on oncological disorders.
- Given the vast potential of AI software and services in clinical studies for improving productivity and research outcomes, many investors have extended financial support; around USD 2.5 billion has been invested till date.
- Over time, several big pharma players have adopted AI software and services in clinical trials to speed up drug discovery and development programs across different therapeutic areas.
- Our proprietary value creation framework highlights the value addition and associated risks of integrating key tools and technologies in clinical trials.
- AI solutions hold significant cost saving potential, along with the ability to expedite trial outcomes and success, across various trial phases.
- The overall opportunity of AI in clinical trials is likely to grow at a CAGR of more than 15%; this opportunity is expected to be well distributed across clinical trial phases, therapeutic areas, end-users and geographical regions.
Table of Contents
- PREFACE
1.1. AI in Clinical Trials Overview
1.2. Key Market Insights
1.3. Scope of the Report
1.4. Research Methodology
1.5. Frequently Asked Questions
1.6. Chapter Outlines
- EXECUTIVE SUMMARY
- INTRODUCTION
3.1. Chapter Overview
3.2. Evolution of AI
3.3. Subfields of AI
3.4. Applications of AI in Healthcare
3.5. Applications of AI in Clinical Trials
3.6. Challenges Associated with the Adoption of AI
3.7. Future Perspective
- MARKET LANDSCAPE
4.1. Chapter Overview
4.2. AI in Clinical Trials: AI Software and Service Providers Landscape
4.2.1. Analysis by Year of Establishment
4.2.2. Analysis by Company Size
4.2.3. Analysis by Location of Headquarters
4.2.4. Analysis by Company Size and Location of Headquarters (Region-wise)
4.2.5. Analysis by Key Offering(s)
4.2.6. Analysis by Business Model(s)
4.2.7. Analysis by Deployment Option(s)
4.2.8. Analysis by Type of AI Technology
4.2.9. Analysis by Application Area(s)
4.2.10. Analysis by Potential End-user(s)
- COMPANY PROFILES
5.1. Chapter Overview
5.2. AiCure
5.2.1. Company Overview
5.2.2. AI-based Clinical Trial Offerings
5.2.3. Recent Developments and Future Outlook
5.3. Antidote Technologies
5.4. Deep 6 AI
5.5. Innoplexus
5.6. IQVIA
5.7. Median Technologies
5.8. Medidata
5.9. Mendel.ai
5.10. Phesi
5.11. Saama Technologies
5.12. Signant Health
5.13. Trials.ai
- CLINICAL TRIAL ANALYSIS
6.1. Chapter Overview
6.2. Scope and Methodology
6.3. AI in Clinical Trials
6.3.1. Analysis by Trial Registration Year
6.3.2. Analysis by Number of Patients Enrolled
6.3.3. Analysis by Trial Phase
6.3.4. Analysis by Trial Status
6.3.5. Analysis by Trial Registration Year and Status
6.3.6. Analysis by Type of Sponsor
6.3.7. Analysis by Patient Gender
6.3.8. Analysis by Patient Age
6.3.9. Word Cloud Analysis: Emerging Focus Areas
6.3.10. Analysis by Target Therapeutic Area
6.3.11. Analysis by Study Design
6.3.12. Most Active Players: Analysis by Number of Clinical Trials
6.3.13. Analysis of Clinical Trials by Geography
6.3.14. Analysis of Clinical Trials by Geography and Trial Status
6.3.15. Analysis of Patients Enrolled by Geography and Trial Registration Year
6.3.16. Analysis of Patients Enrolled by Geography and Trial Status
- PARTNERSHIPS AND COLLABORATIONS
7.1. Chapter Overview
7.2. Partnership Models
7.3. AI in Clinical Trials: Partnerships and Collaborations
7.3.1. Analysis by Year of Partnership
7.3.2. Analysis by Type of Partnership
7.3.3. Analysis by Year and Type of Partnership
7.3.4. Analysis by Application Area
7.3.5. Analysis by Target Therapeutic Area
7.3.6. Analysis by Type of Partner
7.3.7. Most Active Players: Analysis by Number of Partnerships
7.3.8. Analysis by Geography
- FUNDING AND INVESTMENT ANALYSIS
8.1. Chapter Overview
8.2. Types of Funding
8.3. AI in Clinical Trials: Funding and Investments
8.3.1. Analysis by Year of Funding
8.3.2. Analysis by Amount Invested
8.3.3. Analysis by Type of Funding
8.3.4. Analysis by Year and Type of Funding
8.3.5. Analysis by Type of Funding and Amount Invested
8.3.6. Analysis by Application Area
8.6.7. Analysis by Geography
8.3.6. Most Active Players: Analysis by Number of Funding Instances and Amount
Raised
8.3.7. Leading Investors: Analysis by Number of Funding Instances
8.4. Concluding Remarks
- BIG PHARMA INITIATIVES
9.1. Chapter Overview
9.2. Scope and Methodology
9.3. Analysis by Year of Initiative
9.4. Analysis by Type of Initiative
9.5. Analysis by Application Area of AI
9.6. Analysis by Target Therapeutic Area
9.7. Benchmarking Analysis: Big Pharma Players
- AI IN CLINICAL TRIALS: USE CASES
10.1. Chapter Overview
10.2. Use Case 1: Collaboration between Roche and AiCure
10.2.1. Roche
10.2.2. AiCure
10.2.3. Business Needs
10.2.4. Objectives Achieved and Solutions Provided
10.3. Use Case 2: Collaboration between Takeda and AiCure
10.4. Use Case 3: Collaboration between Teva Pharmaceuticals and Intel
10.5. Use Case 4: Collaboration between Undisclosed Pharmaceutical Company and Antidote
10.6. Use Case 5: Collaboration between Undisclosed Pharmaceutical Company and Cognizant
10.7. Use Case 6: Collaboration between Cedars-Sinai Medical Center and Deep 6 AI
10.8. Use Case 7: Collaboration between GlaxoSmithKline (GSK) and PathAI
10.9. Use Case 8: Collaboration between Bristol Myers Squibb (BMS) and Concert AI
- VALUE CREATION FRAMEWORK: A STRATEGIC GUIDE TO ADDRESS UNMET NEEDS IN CLINICAL TRIALS
11.1. Chapter Overview
11.2. Unmet Needs in Clinical Trials
11.3. Key Assumptions and Methodology
11.4. Key Tools and Technologies
11.4.1. Blockchain
11.4.2. Big Data Analytics
11.4.3. Real-world Evidence
11.4.4. Digital Twins
11.4.5. Cloud Computing
11.4.6. Internet of Things (IoT)
11.5. Trends in Research Activity
11.6. Trends in Intellectual Capital
11.7. Extent of Innovation versus Associated Risks
11.8. Results and Discussion
- COST SAVING ANALYSIS
12.1. Chapter Overview
12.2. Key Assumptions and Methodology
12.3. Overall Cost Saving Potential of AI in Clinical Trials, 2023-2035
12.4. Conclusion
- MARKET FORECAST AND OPPORTUNITY ANALYSIS
13.1. Chapter Overview
13.2. Key Assumptions and Forecast Methodology
13.3. Global AI in Clinical Trials Market, 2018-2035
13.3.1. AI in Clinical Trials Market: Distribution by Trial Phase, 2023 and 2035
13.3.2. AI in Clinical Trials Market: Distribution by Target Therapeutic Area, 2023 and 2035
13.3.3. AI in Clinical Trials Market: Distribution by End-user, 2023 and 2035
13.3.4. AI in Clinical Trials Market: Distribution by Key Geographical Regions, 2023 and 2035
- CONCLUSION
- EXECUTIVE INSIGHTS
15.1. Chapter Overview
15.2. Ancora.ai
15.3. Deep 6 AI
15.4. Intelligencia
15.5. nQ Medical
15.6. Science 37
- APPENDIX I: TABULATED DATA
- APPENDIX II: LIST OF COMPANIES AND ORGANIZATIONS
To view more details on this report, click on the link
https://www.rootsanalysis.com/reports/ai-based-clinical-trial-solutions.html
News article
AI-based Digital Pathology Market
Learn from experts: do you know about these emerging industry trends?
Cell Free System market: Current Landscape, Growth Opportunities and Future Market Outlook
About Roots Analysis
Roots Analysis is a global leader in the pharma / biotech market research. Having worked with over 750 clients worldwide, including Fortune 500 companies, start-ups, academia, venture capitalists and strategic investors for more than a decade, we offer a highly analytical / data-driven perspective to a network of over 450,000 senior industry stakeholders looking for credible market insights.
Learn more about Roots Analysis consulting services:
Contact:
Ben Johnson
+1 (415) 800 3415