The Growing Threat: Navigating the Ventilator-Associated Pneumonia (VAP) Market
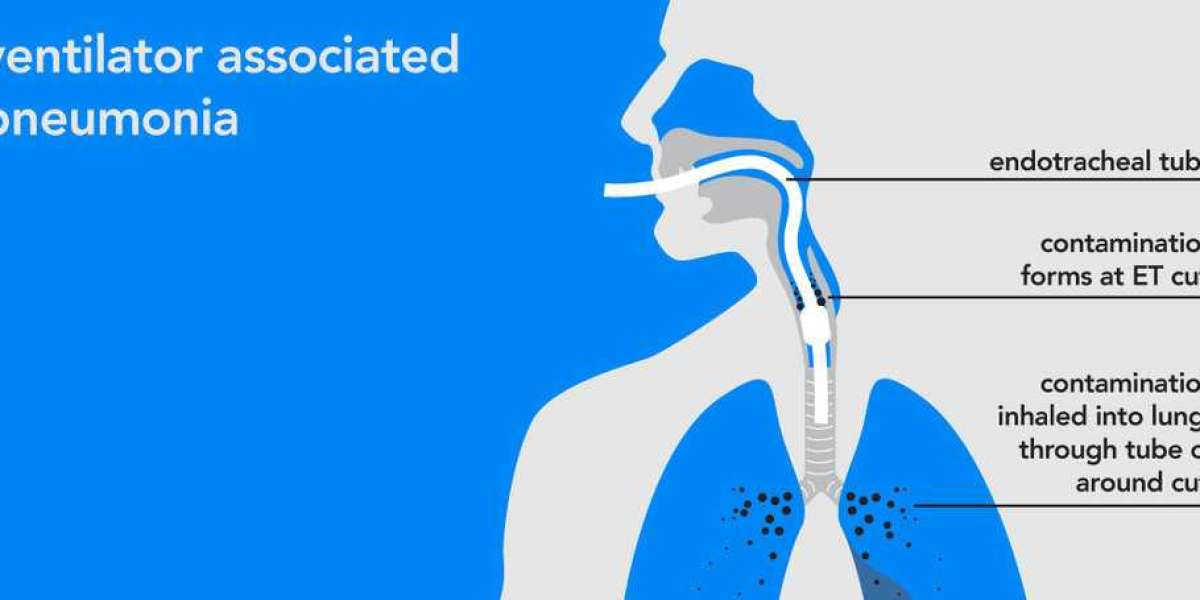
Ventilator-associated pneumonia (VAP) is a serious lung infection that can develop in patients on mechanical ventilation. This life-threatening condition significantly burdens healthcare systems worldwide, leading to increased mortality, morbidity, and financial strain. The Ventilator-Associated Pneumonia (VAP) Market caters to the diagnosis, prevention, and treatment of VAP, and is anticipated to witness significant growth in the coming years.
Market growth is driven by several factors. The rising number of ventilator-dependent patients due to an aging population, chronic respiratory illnesses, and the growing use of critical care interventions are major drivers. Additionally, increasing awareness regarding VAP prevention strategies, advancements in diagnostic tools, and the development of novel VAP therapeutics are propelling market expansion. Furthermore, rising government initiatives to combat hospital-acquired infections and a growing research focus on developing effective VAP treatments contribute to market growth.
The VAP market share is expected to be dominated by North America due to the high prevalence of ventilator-dependent patients, advanced healthcare infrastructure, and significant investments in VAP research. The US is a major contributor within the North American market, driven by factors like a large patient population, high healthcare expenditure, and a strong focus on adopting new technologies. However, the Asia-Pacific region presents the fastest growth potential due to a burgeoning aging population, increasing government focus on improving critical care facilities, and rising healthcare expenditure. China and India are expected to be the key growth drivers within the Asia-Pacific market.
Rapid VAP Diagnostics: A Critical Need
Early and accurate diagnosis of VAP is crucial for effective treatment and improved patient outcomes. Traditional diagnostic methods for VAP can be time-consuming and invasive. However, the field of rapid VAP diagnostics is witnessing significant advancements. These advancements involve the development of novel diagnostic tools that offer faster and more accurate VAP detection.
One promising area of exploration is the use of molecular diagnostics for VAP. Molecular diagnostic tests can identify specific pathogens responsible for VAP infection, allowing for targeted antibiotic therapy. This not only improves patient outcomes but also helps combat the rise of antibiotic-resistant bacteria. Additionally, research is ongoing on the development of non-invasive diagnostic methods like point-of-care tests. These tests can be performed at the bedside, further streamlining the VAP diagnosis process and enabling quicker treatment initiation.
The development of rapid VAP diagnostics holds immense promise for the VAP market. These innovative tools can significantly improve VAP management, leading to better patient outcomes and reduced healthcare costs. As these advancements continue to gain traction, the VAP market is poised to experience significant transformation.
Recent Technological Advancements and Investments by Leading Companies
The VAP market is witnessing a surge in innovation, with key players actively developing and commercializing novel solutions for VAP diagnosis, prevention, and treatment. Here's a glimpse into some recent developments and investments by leading companies:
- Adenium Biotech ApS (Denmark): In April 2024, Adenium announced positive pre-clinical results for its groundbreaking Adnectin™-based therapy for VAP. This innovative therapy utilizes Adnectin™ molecules, small and versatile proteins, to target specific components of bacteria responsible for VAP. This targeted approach holds promise for offering a more effective and potentially less toxic treatment option compared to traditional broad-spectrum antibiotics.
- Crdeas Pharma (US): Crdeas is currently in Phase II clinical trials for its revolutionary VAP diagnostic test. This rapid point-of-care test leverages a multi-analyte immunoassay platform to detect various biomarkers indicative of VAP infection. This test has the potential to significantly reduce diagnosis time compared to traditional methods, enabling quicker treatment initiation and improved patient outcomes.
- Merck Co., Inc. (US): Merck, a well-established pharmaceutical giant, continues to invest heavily in research and development of new VAP antibiotics. In April 2024, Merck announced a collaboration with a leading academic institution to explore the potential of novel antibiotic candidates targeting emerging drug-resistant strains associated with VAP. This collaboration highlights Merck's commitment to addressing the growing challenge of antibiotic resistance in VAP treatment.
- Shinogi Inc. (Japan): Shinogi is actively exploring the development of novel VAP therapeutics with unique mechanisms of action. In March 2024, Shinogi announced a strategic partnership with a biotechnology company to develop a new class of anti-virulence agents targeting bacterial virulence factors associated with VAP pathogenesis. This approach aims to disarm bacteria's ability to cause infection, potentially leading to more effective VAP treatment strategies.
The VAP market presents a dynamic landscape with immense growth potential. Rising healthcare expenditure, increasing awareness about VAP, and continuous technological advancements are propelling market expansion. As leading companies invest in novel VAP diagnostics, therapeutics, and preventative measures, the future of VAP management appears promising. The combined efforts of these companies offer hope for a future with reduced VAP incidence rates, improved patient outcomes, and a more effective healthcare response to this critical condition.
For more information visit at MarketResearchFuture
Other Trending Reports